
本日eduroam 認証サーバのサーバ証明書を更新いたします。
ユーザ端末から最初にeduroamに接続した際、更新後の証明書を信頼するように求められることがあります。
以下の情報を確認の上、接続を行ってください。
発行先:edrad.sic.shibaura-it.ac.jp
発行元:NII Open Domain CA - G7 RSA
サーバーの拇印:ba 5d 5f 7f dc 06 19 84 ea da 55 f2 c1 b5 6c cd db 83 56 69
eduroamの利用方法についてはこちらのページをご確認下さい。
学内DBサーバ更新作業の影響で、現在一部のユーザにてStationログインエラーが発生しております。
以下のメッセージが表示される場合、お手数ですがブラウザのキャッシュをクリア、もしくはプライベートウィンドウを使用してのログインをお試し下さい。
Couldn't get your session object. Please contant to system administrator.
※上記方法でログイン出来ない場合でも、何度か繰り返すことでログイン出来ます。
ご利用の皆様にはご不便をおかけいたしますが、何卒よろしくお願いいたします。
eduroamサーバの入れ替えに伴い、学内・学外でeduroamサービスが利用できない時間帯が発生いたします。
利用停止時間:3/16(土) 12:00-13:00の間で10分程度
ご利用の皆様にはご不便をおかけいたしますが、何卒よろしくお願いいたします。
各キャンパスでは、土曜日は情報イノベーション課の窓口閉室後も
以下の時間帯でPC実習室を利用することができます。
大宮キャンパス 5号館1F 情報処理教室 17時~20時30分
豊洲キャンパス 教室棟6F PC実習室6 17時~20時30分
詳細につきましてはこちらのページをご確認ください。
PC実習室時間外利用
※4/23 11:00 実施日時更新
以下日時において、MicorosoftによるAzureDBのメンテナンスを行います。
2024年4月24日(水)午前1時~午前2時
2024年4月27日(土)午前4時16分~午前5時16分
メンテナンス中は接続不良が発生します。テストの実施や課題の追加などはお控えください。
ご不便をおかけして大変申し訳ございませんが、ご協力の程何卒よろしくお願いいたします
以下日時において、データベースサーバ移行のためのメンテナンスを行います。
2024年3月28日(木)15時~21時
メンテナンス中は接続不良が発生します。
テストの実施や課題の追加などはお控えください。
ご不便をおかけして大変申し訳ございませんが、
ご協力の程何卒よろしくお願いいたします。
以下日時において、MicorosoftによるAzureDBのメンテナンスを行います。
2024年3月22日(金)17時~3月23日(土)8時
メンテナンス中は接続不良が発生します。
テストの実施や課題の追加などはお控えください。
ご不便をおかけして大変申し訳ございませんが、
ご協力の程何卒よろしくお願いいたします。
以下日時において、MicorosoftによるAzureDBのメンテナンスを行います。
2023年10月25日(水)20時~21時
メンテナンス中は接続不良が発生します。
テストの実施や課題の追加などはお控えください。
ご不便をおかけして大変申し訳ございませんが、
ご協力の程何卒よろしくお願いいたします。
以下日時において、MicorosoftによるAzureDBのメンテナンスを行います。
2023年10月24日(火)17時~10月25日(水)8時
メンテナンス中は接続不良が発生します。
テストの実施や課題の追加などはお控えください。
ご不便をおかけして大変申し訳ございませんが、
ご協力の程何卒よろしくお願いいたします。
学生・教職員のみなさま
情報システム課からのお知らせです。
インターネットの商用サービスや、本学のアカウント情報の詐取を目的としたフィッシングメールが増えています。
一部はメールセキュリティシステムをすり抜けて配信されてくる場合があります。
利用者から不審なメールが届いたとの報告が寄せられています。
不審なメールが届いた場合は、本文中のURLにアクセスをしないよう気を付けてください。
添付ファイルが付いている場合も、開かないようにしてください。
※送信者(From:)は、詐称されている事が多く、自身のメールアドレスや、管理者のメールアドレス(admin@~等)となっている場合もあります。
件名の一例:
【重要】【共用】【2023年7月13日(木曜日) (JST)】サーバーメンテナンスのお知らせ
sic.shibaura-it.ac.jp 新Webメールの提供開始
mail delivery failure: storage is almost full
ow.shibaura-it.ac.jp mail service update Case ID: ?????
You have [13] pending incoming emails
SYSTEM NOTIFICATION ~ Your 8 unreceived emails are stuck on the sic.shibaura-it.ac.jp Server.
Your ow.shibaura-it.ac.jp Account will be disabled soonest.
Your Outlook storage is full
Verify User
Mail Notice: De-activation
Account Termination
Email Termintaion Request
Undeliverable: Incoming messages failure
Check Password
Email Verification ~ VERIFY YOUR EMAIL ACCOUNT TODAY
~: Unknown Login Notification
Password Expiration Notice!!!
~ 電子メール通知
必要なアクション:~ のメール非アクティブ化通知
必要なアクション Tuesday, June 27, 2023 の重要な通知
必要なアクション Monday, June 26, 2023 の重要な通知
必要なアクション Sunday, June 25, 2023 の重要な通知
あなたが持っている 新しいボイスメールメモを受信しました.
メール受信エラー
お知らせ: メールボックスのサイズがクォータ制限に達しました
Sic メールボックスのサイズがクォータ制限に達しました
メールボックスのストレージ容量が少ない
~ 受信メールエラー
メールボックスのストレージがいっぱいです、アカウントが一時停止されています
不審なメールを受信した場合は、 SIT-CSIRTまでご連絡ください。
SIT-CSIRT (芝浦工業大学 Computer Security Incident Response Team)
Email: csirt@shibaura-it.ac.jp
検索サイトから本学に関連するキーワードを検索すると、本学を騙る偽サイトが検索結果に表示されることを確認いたしました。
本件に限らず、最近検索結果に偽サイトが表示されることが散見されます。
偽サイトの中にはアクセスをするとWebブラウザに感染するものや、マルウエアがダウンロードされるものも存在しますので、常に検索結果に不審なものが含まれていないか確認することを心掛けてください。
また、偽サイトを発見した場合は、SIT-CSIRTまでご報告いただけます様お願いいたします。
SIT-CSIRT
csirt@shibaura-it.ac.jp
We have confirmed that a fake website impersonating our university appears in the search results when you search for keywords related to our university through search engines.
Not only in this case, but also in other recent cases, fake sites have been appearing in search results.
Some of the fake sites may infect web browsers or download malware when accessed, so please be sure to always check your search results for suspicious items.
If you find a fake site, please report it to SIT-CSIRT.
SIT-CSIRT
csirt@shibaura-it.ac.jp
検索結果の例
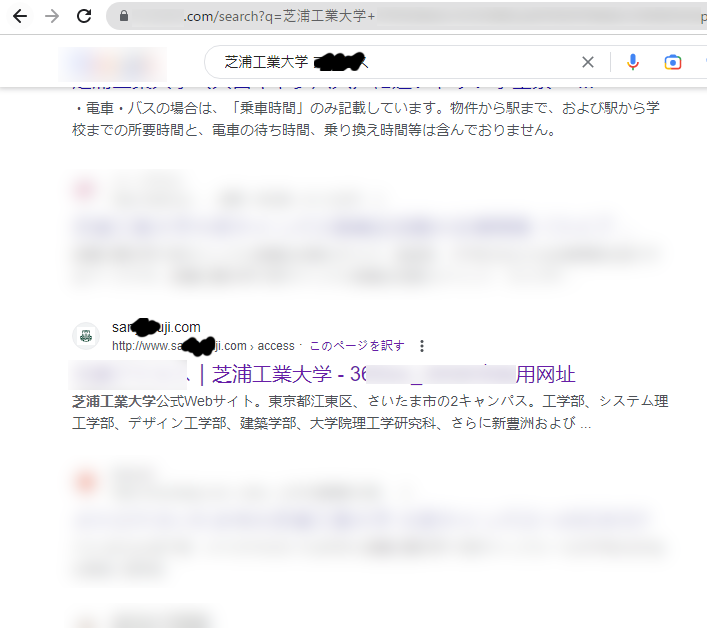 |
偽サイトの例
 |
Emotetは、メールに添付されたWordやExcelのファイルを開くことで感染することが多いマルウエアです。
(2023/3/16追記)
本日より、添付ファイルの形式がOneNote (拡張子.one)になっているようです。不審な添付ファイルは開かないようご注意ください。
(2023/3/08追記)
2022年11月に一時的に再開し、再度活動が停止していましたが、2023/3/7より、再度メールの送信が行われております。
日本語の文面や、偽装返信型も出ているという情報がありますので、ご注意ください。
本日の時点で、添付ファイルはzip形式で、zipファイルを展開すると500M以上の大きな.docファイルになっており、Wordのマクロ型です。
今後添付ファイルの形式等が変わる可能性もありますのでご注意ください。
(2022/11/02追記)
2022/7/13頃より活動が停止していましたが、2022/11/2より活動が再開し、感染を引き起こすメールが大量に送信されています。
マルウェアEmotetの感染再拡大に関する注意喚起 (JPCERT/CC)
2021/1/27にEUROPOL(欧州刑事警察機構)により、差し押さえが行われ停止いたしましたが、2021/11/14頃より、活動が再開しました。
添付ファイルは、WordやExcelファイル以外にも、パスワード付きzipファイルのものも出回っております。
Emotetに感染すると、OutlookやThunderbirdのメール情報(メールサーバへの認証情報や受信箱のメールアドレス、件名等)を窃取されるだけでなく、他のマルウエア(CobaltStrikeやQbot、TrickBot、Ryukランサムウエア、Ursnifバンキングマルウエア等)に追加感染することがあります。
窃取されたメール情報は、感染拡大のためのメールのばら撒きに利用されることがあります。
メールの件名は様々あり、窃取されたメールに対する返信の形をとるものもありますので、注意が必要です。
このようなメールが届いた場合は、絶対に開かずに、SIT-CSIRTまでご連絡をお願いいたします。
万一開いてしまい、コンテンツの有効化をしてしまった場合は、すぐにネットワークから切断してください。
ご利用の端末のアンチウイルスソフトが正しく動作しているかどうかご確認をお願いいたします。
メールの送信者・宛先・メール本文の署名欄が自身の名前になっている場合は、自身もしくは過去にメールを送受信した人が感染した可能性が考えられます。
自身のPCが感染しているか不安な方は、感染をチェックするツール「EmoCheck」をお試しください。
Emotet感染確認ツール「EmoCheck2.0」の実行手順(警視庁サイバーセキュリティ対策本部)
不審なメールを受信した場合は、 SIT-CSIRTまでご連絡ください。
SIT-CSIRT (芝浦工業大学 Computer Security Incident Response Team)
Email: csirt@shibaura-it.ac.jp
ばら撒かれるメールには、窃取したメールを本文に引用して返信する形をとるもの(返信型)や、件名や本文の署名に窃取した名前・メールアドレス等を埋め込んであるものが存在しますので注意してください
添付ファイルが無く、メール本文内にリンクが書かれているものも確認されています。リンクをクリックするとEmotetダウンローダのWordファイル等がダウンロードされますので、リンク先にはアクセスしないようご注意願います。
教職員各位
情報システム課からのお知らせです。
年末年始はシステム管理体制が手薄になる事から、セキュリティイ
特に休暇明けは電子メールの確認量が増えることから、不審なメー
近年、日本国内の学術関係者、シンクタンク研究員、報道関係者等
講演依頼や取材依頼等を装ったメールをやりとりする中で不正なプ
当該人物のやりとりするメールやコンピュータ内のファイルの内容
詳細は、警察庁サイバー犯罪対策プロジェクトの情報をご覧くださ
( https://www.npa.go.jp/cyber/ )
また、本学では専任教員・事務職員向けに電子メールの標的型攻撃
不審なメールは自動的に隔離され、毎日15:00に通知メールが
https://web.sic.shibaura-it.a
不審なメールを受信した場合は、 SIT-CSIRTまでご連絡ください。
SIT-CSIRT (芝浦工業大学 Computer Security Incident Response Team)
Email: csirt@shibaura-it.ac.jp
学生・教職員のみなさま
情報システム課からのお知らせです。
本日複数の方からEmotetマルウエアの不審なメールを受信したとの通報が多く寄せられております。
昨年11/25日にお知らせいたしましたが、再度ご確認をお願いいたします。
学術情報センターホームページのセキュリティ情報にも掲載してありますので、あわせてご確認ください。
1. フィッシングメールに対する注意のお願い
最近Webメールサービスのアカウント情報の詐取を目的としたフィッシングメールの被害が多発しております。
本学の利用者からも、不審なメールが届いたとの報告が寄せられています。
不審なメールが届いた場合は、本文中のURLにアクセスをしないよう気を付けてください。
添付ファイルが付いている場合も、開かないようにしてください。
※ 学術情報センターホームページのセキュリティ情報をご確認ください。
件名の一例:
メールプラン、セキュリティ強化第2弾リリースのお知らせ
【重要】2021年XX月XX日(X)のサービス復旧のお知らせ。
【重要】使用制限によるサービス停止のお知らせ- {メールアドレス}
【重要】サービス停止のお知らせ - {メールアドレス}
【お知らせ】メール配信通知 - {メールアドレス}
[メール接続サービスの終了について_shibaura-it.ac.jp] - {メールアドレス}
[からの通知_Shibaura-it]_{メールアドレス}
【バージョンアップ】メンテナンス作業のお知らせ
【重要】sic.shibaura-it.ac.jp メール送信機能停止のお知らせ
不審なメールを受信した場合は、 SIT-CSIRTまでご連絡ください。
SIT-CSIRT (芝浦工業大学 Computer Security Incident Response Team)
Email: csirt@shibaura-it.ac.jp
2. Emotetマルウエアメールに対する注意のお願い
Emotetマルウエアは、2021/1/27にEUROPOL(欧州刑事警察機構)により、差し押さえが行われ停止いたしましたが、
2021/11/14頃より、活動再開の兆候があります。
日本をターゲットにしたメールも送信されているようです。
※ Emotetマルウエアに関する情報も、学術情報センターホームページのセキュリティ情報をご確認ください。
不審なメールを受信した場合は、 SIT-CSIRTまでご連絡ください。
SIT-CSIRT (芝浦工業大学 Computer Security Incident Response Team)
Email: csirt@shibaura-it.ac.jp
3. ウェブサーバのCMSへの攻撃に関する注意のお願い
研究室等でウェブサーバーを運用されており、CMS (WordPress、Movable Type等)を利用されている場合は、 不審なアクセスがないかご確認ください。
国内において、不正なコンテンツの埋め込みや、フィッシングメールの大量送信、サーバーへの不正侵入等の被害が発生しております。
利用しているウェブサーバーのパスワードの管理の徹底、脆弱性のあるソフトウエアの更新、不要なサービスの停止等の対策をお願いいたします。
不審なアクセスを発見した場合はSIT-CSIRTまでご連絡ください。
SIT-CSIRT (芝浦工業大学 Computer Security Incident Response Team)
Email: csirt@shibaura-it.ac.jp
| 日 | 月 | 火 | 水 | 木 | 金 | 土 |
31 1 | 1 | 2 | 3 | 4 | 5 | 6 |
7 1 | 8 | 9 | 10 | 11 | 12 | 13 |
14 1 | 15 | 16 | 17 | 18 | 19 | 20 |
21 1 | 22 | 23 | 24 | 25 | 26 | 27 |
28 1 | 29 1 | 30 1 | 1 1 | 2 1 | 3 1 | 4 1 |